iConcur app for iPhone and iPad
Developer: Mylan Inc.
First release : 29 Jul 2015
App size: 18.12 Mb
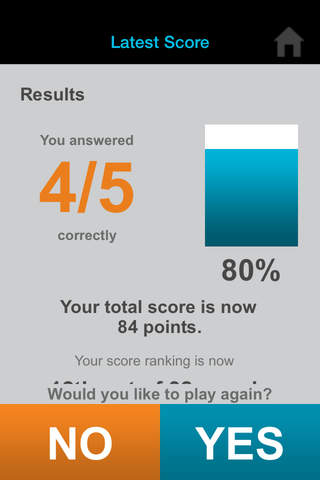
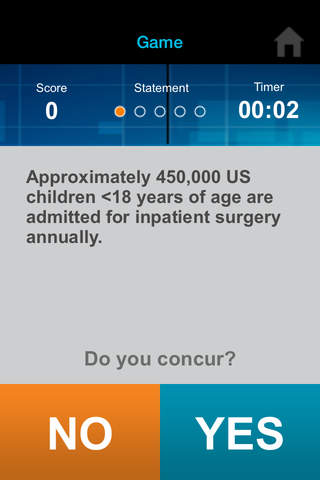
Can you outscore your colleagues? Find out with iConcur, a new game app from Mylan Speciality L.P., manufacturer of ULTIVA® (remifentanil HCl) for Injection.
• Agree or disagree with quiz statements as quickly as you can.
• Test your knowledge and quickness in anesthesia and trivia.
• See rankings from all players and compete with colleagues in your office or nationwide.
THIS APP IS INTENDED ONLY FOR ANESTHESIA PROVIDERS IN THE UNITED STATES. USE OF ANY DRUG, THERAPEUTIC REGIMEN, OR MEDICAL TECHNIQUE MENTIONED IN THIS APP
IS SUBJECT TO THE INDEPENDENT KNOWLEDGE AND JUDGMENT OF THE PRACTITIONER. DO NOT USE THIS APP IN ANY ARENA WHERE ACTIVE CLINICAL CARE IS OCCURRING.
INDICATIONS AND IMPORTANT RISK INFORMATION
INDICATIONS
ULTIVA® (remifentanil HCl) for Injection is indicated for intravenous
administration:
• As an analgesic agent for use during the induction and maintenance of general anesthesia for inpatient and outpatient procedures
• For continuation as an analgesic into the immediate postoperative period in adult patients under the direct supervision of an anesthesia practitioner in a postoperative anesthesia care unit or intensive care setting
• As an analgesic component of monitored anesthesia care in adult patients
IMPORTANT RISK INFORMATION
Due to the presence of glycine in the formulation, ULTIVA is contraindicated for epidural or intrathecal administration. ULTIVA is also contraindicated in patients with known hypersensitivity to
fentanyl analogs.
Vital signs and oxygenation must be continuously monitored during ULTIVA administration. ULTIVA produces adverse events that are characteristic of μ-opioids, such as respiratory depression, apnea,
tachycardia, bradycardia, hypotension, hypertension, and skeletal muscle (including chest wall) rigidity. Because these effects are dose-dependent and can occur rapidly, continuous monitoring is
necessary. ULTIVA should not be used as a sole agent because loss of consciousness cannot be assured and because of a high incidence of apnea, muscle rigidity, and tachycardia.
Continuous infusions of ULTIVA should be administered only by an infusion device. IV bolus administration of ULTIVA should be used only during the maintenance of general anesthesia.
In nonintubated patients, single doses of ULTIVA should be administered over 30 to 60 seconds. Interruption of an infusion of ULTIVA will result in rapid offset of effect. Rapid clearance and lack
of drug accumulation result in rapid dissipation of respiratory depressant and analgesic effects (within 5 to 10 min) upon discontinuation of ULTIVA at recommended doses. Discontinuation of
an infusion of ULTIVA should be preceded by the establishment of adequate postoperative analgesia particularly where postoperative pain is anticipated.
ULTIVA should be used with caution in pediatric, geriatric, and morbidly obese patients due to high variability in pharmacodynamics and dose/response. Intraoperative awareness has been reported with concomitant administration with propofol infusion ≤75 mcg/kg/min.
Failure to adequately clear the IV tubing to remove residual ULTIVA has been associated with the appearance of respiratory depression, apnea, and muscle rigidity upon the administration of additional fluids or medications through the same IV tubing.
ULTIVA SHOULD BE USED IN CAREFULLY MONITORED SETTINGS BY SPECIFICALLY TRAINED PERSONS NOT INVOLVED IN THE SURGICAL OR DIAGNOSTIC PROCEDURE. OXYGEN SATURATION IS TO BE CONTINUOUSLY MONITORED. RESUSCITATIVE AND INTUBATION EQUIPMENT, OXYGEN, AND AN OPIOID ANTAGONIST MUST BE READILY AVAILABLE.
Please visit www.ultiva.com to download the ULTIVA full Prescribing Information for all indications, precautions, warnings, contraindications, and adverse events.
If you have questions or comments, please call us at 800.RX.MYLAN.
ULTIVA is a registered trademark of Glaxo Group Limited. The Mylan logo is a registered trademark of Mylan Inc.